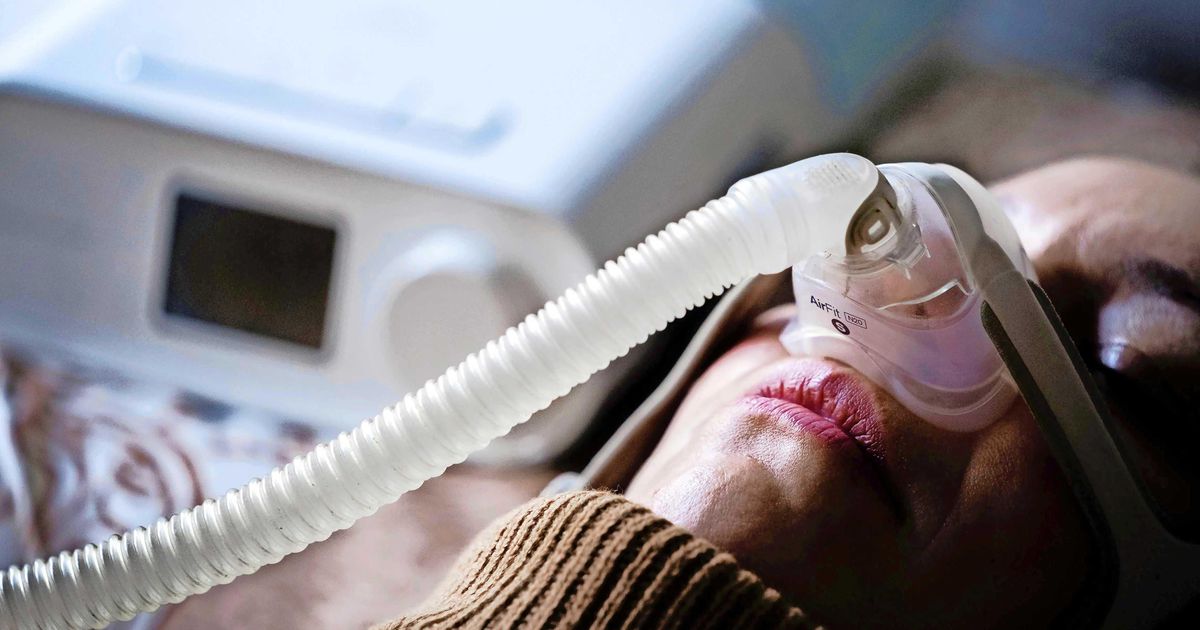
The U.S. regulator, the FDA, warns against this, ordering the Dutch company to notify all patients, suppliers and physicians concerned with the equipment in question regarding technical issues. Phillips recalled millions of sleep breathing devices last year because the insulating foam would shatter and be detrimental to health.
⁇
The FDA is aware of the frustration among patients and medical device suppliers who are unaware of the withdrawal and has not received enough information about the next steps in the withdrawal process, “said Jeff Shuren, director of the FDA’s medical devices division on the supervisor’s website.
Philips began recalling about 5.2 million devices worldwide in June 2021. The regulator says it has warned the technology company in advance that information about the dangers of sleep apnea devices has not reached everyone.
Philips said in its own newsletter that it had reached “most users or suppliers” of sleep apnea devices in the United States in two sessions. About 2.6 million devices are now registered. Philips also sends alerts via mobile app to patients with sleep apnea.
The FDA has previously sued Phillips over the recall of sleep apnea devices. The U.S. Food and Consumer Product Safety Commission wrote last fall that Philips should have known in advance that there were issues with the products, but failed to investigate further after the complaints were reported.
Philips has booked அழைக்க 725 million for the recall. There may also be claims for damages.